MAST2
Protein-coding gene in the species Homo sapiens
| MAST2 | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | MAST2, MAST205, MTSSK, microtubule associated serine/threonine kinase 2 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 612257 MGI: 894676 HomoloGene: 7428 GeneCards: MAST2 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
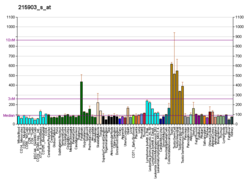
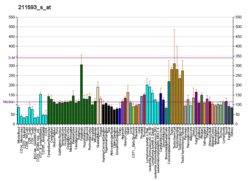
Microtubule-associated serine/threonine-protein kinase 2 is an enzyme that in humans is encoded by the MAST2 gene.[5] The protein encoded by this gene controls TRAF6 and NF-kappaB activity.[6]
Interactions
MAST2 has been shown to interact with PCLKC.[7]
References
- ^ a b c GRCh38: Ensembl release 89: ENSG00000086015 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000003810 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Entrez Gene: MAST2 microtubule associated serine/threonine kinase 2".
- ^ Xiong H, Li H, Chen Y, Zhao J, Unkeless JC (2004). "Interaction of TRAF6 with MAST205 regulates NF-kappaB activation and MAST205 stability". J. Biol. Chem. 279 (42): 43675–83. doi:10.1074/jbc.M404328200. PMID 15308666.
- ^ Okazaki N, Takahashi N, Kojima S, Masuho Y, Koga H (July 2002). "Protocadherin LKC, a new candidate for a tumor suppressor of colon and liver cancers, its association with contact inhibition of cell proliferation". Carcinogenesis. 23 (7): 1139–48. doi:10.1093/carcin/23.7.1139. PMID 12117771.
Further reading
- Walden PD, Cowan NJ (1993). "A novel 205-kilodalton testis-specific serine/threonine protein kinase associated with microtubules of the spermatid manchette". Mol. Cell. Biol. 13 (12): 7625–35. doi:10.1128/mcb.13.12.7625. PMC 364834. PMID 8246979.
- Bonaldo MF, Lennon G, Soares MB (1996). "Normalization and subtraction: two approaches to facilitate gene discovery". Genome Res. 6 (9): 791–806. doi:10.1101/gr.6.9.791. PMID 8889548.
- Walden PD, Millette CF (1996). "Increased activity associated with the MAST205 protein kinase complex during mammalian spermiogenesis". Biol. Reprod. 55 (5): 1039–44. doi:10.1095/biolreprod55.5.1039. PMID 8902215.
- Nagase T, Ishikawa K, Suyama M, Kikuno R, Miyajima N, Tanaka A, Kotani H, Nomura N, Ohara O (1998). "Prediction of the coding sequences of unidentified human genes. XI. The complete sequences of 100 new cDNA clones from brain which code for large proteins in vitro". DNA Res. 5 (5): 277–86. doi:10.1093/dnares/5.5.277. PMID 9872452.
- Lumeng C, Phelps S, Crawford GE, Walden PD, Barald K, Chamberlain JS (1999). "Interactions between beta 2-syntrophin and a family of microtubule-associated serine/threonine kinases". Nat. Neurosci. 2 (7): 611–7. doi:10.1038/10165. PMID 10404183. S2CID 20910888.
- Adey NB, Huang L, Ormonde PA, Baumgard ML, Pero R, Byreddy DV, Tavtigian SV, Bartel PL (2000). "Threonine phosphorylation of the MMAC1/PTEN PDZ binding domain both inhibits and stimulates PDZ binding". Cancer Res. 60 (1): 35–7. PMID 10646847.
- Gisler SM, Stagljar I, Traebert M, Bacic D, Biber J, Murer H (2001). "Interaction of the type IIa Na/Pi cotransporter with PDZ proteins". J. Biol. Chem. 276 (12): 9206–13. doi:10.1074/jbc.M008745200. PMID 11099500.
- Okazaki N, Takahashi N, Kojima S, Masuho Y, Koga H (2002). "Protocadherin LKC, a new candidate for a tumor suppressor of colon and liver cancers, its association with contact inhibition of cell proliferation". Carcinogenesis. 23 (7): 1139–48. doi:10.1093/carcin/23.7.1139. PMID 12117771.
- Nakayama M, Kikuno R, Ohara O (2002). "Protein-protein interactions between large proteins: two-hybrid screening using a functionally classified library composed of long cDNAs". Genome Res. 12 (11): 1773–84. doi:10.1101/gr.406902. PMC 187542. PMID 12421765.
- Navarro-Lérida I, Martínez Moreno M, Roncal F, Gavilanes F, Albar JP, Rodríguez-Crespo I (2004). "Proteomic identification of brain proteins that interact with dynein light chain LC8". Proteomics. 4 (2): 339–46. doi:10.1002/pmic.200300528. PMID 14760703. S2CID 8868600.
- Zhou H, Xiong H, Li H, Plevy SE, Walden PD, Sassaroli M, Prestwich GD, Unkeless JC (2004). "Microtubule-associated serine/threonine kinase-205 kDa and Fc gamma receptor control IL-12 p40 synthesis and NF-kappa B activation". J. Immunol. 172 (4): 2559–68. doi:10.4049/jimmunol.172.4.2559. PMID 14764729. S2CID 85692192.
- Brandenberger R, Wei H, Zhang S, Lei S, Murage J, Fisk GJ, Li Y, Xu C, Fang R, Guegler K, Rao MS, Mandalam R, Lebkowski J, Stanton LW (2004). "Transcriptome characterization elucidates signaling networks that control human ES cell growth and differentiation". Nat. Biotechnol. 22 (6): 707–16. doi:10.1038/nbt971. PMID 15146197. S2CID 27764390.
- Xiong H, Li H, Chen Y, Zhao J, Unkeless JC (2004). "Interaction of TRAF6 with MAST205 regulates NF-kappaB activation and MAST205 stability". J. Biol. Chem. 279 (42): 43675–83. doi:10.1074/jbc.M404328200. PMID 15308666.
- Olsen JV, Blagoev B, Gnad F, Macek B, Kumar C, Mortensen P, Mann M (2006). "Global, in vivo, and site-specific phosphorylation dynamics in signaling networks". Cell. 127 (3): 635–48. doi:10.1016/j.cell.2006.09.026. PMID 17081983. S2CID 7827573.
- Ewing RM, Chu P, Elisma F, Li H, Taylor P, Climie S, McBroom-Cerajewski L, Robinson MD, O'Connor L, Li M, Taylor R, Dharsee M, Ho Y, Heilbut A, Moore L, Zhang S, Ornatsky O, Bukhman YV, Ethier M, Sheng Y, Vasilescu J, Abu-Farha M, Lambert JP, Duewel HS, Stewart II, Kuehl B, Hogue K, Colwill K, Gladwish K, Muskat B, Kinach R, Adams SL, Moran MF, Morin GB, Topaloglou T, Figeys D (2007). "Large-scale mapping of human protein-protein interactions by mass spectrometry". Mol. Syst. Biol. 3 (1): 89. doi:10.1038/msb4100134. PMC 1847948. PMID 17353931.
- v
- t
- e
Serine/threonine-specific protein kinases (EC 2.7.11.21-EC 2.7.11.30) | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||
Dual-specificity kinases (EC 2.7.12) | |||
|---|---|---|---|
| |||