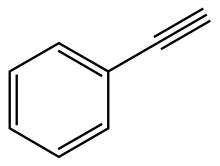
Phenylacetylene
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name Ethynylbenzene | |
| Other names Phenylacetylene | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEMBL |
|
| ChemSpider |
|
| ECHA InfoCard | 100.007.861 |
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
InChI
| |
| |
| Properties | |
Chemical formula | C8H6 |
| Molar mass | 102.133 g/mol |
| Density | 0.93 g/cm3 |
| Melting point | −45 °C (−49 °F; 228 K) |
| Boiling point | 142 to 144 °C (288 to 291 °F; 415 to 417 K) |
| Acidity (pKa) | 28.7 (DMSO),[1] 23.2 (aq, extrapolated)[2] |
Magnetic susceptibility (χ) | -72.01·10−6 cm3/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).  Y verify (what is Y verify (what is  Y Y N ?) N ?) Infobox references | |
Phenylacetylene is an alkyne hydrocarbon containing a phenyl group. It exists as a colorless, viscous liquid. In research, it is sometimes used as an analog for acetylene; being a liquid, it is easier to handle than acetylene gas.
Preparation
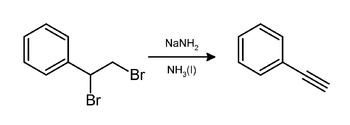
In the laboratory, phenylacetylene can be prepared by elimination of hydrogen bromide from styrene dibromide using sodium amide in ammonia:[3]
It can also be prepared by the elimination of hydrogen bromide from bromostyrene using molten potassium hydroxide.[4] Yet another method involves the Sonogashira coupling of iodobenzene with trimethylsilylacetylene, followed by removal of the trimethylsilyl group using TBAF.[5]
Reactions
Phenylacetylene is a prototypical terminal acetylene, undergoing many reactions expected of that functional group. It undergoes semihydrogenation over Lindlar catalyst to give styrene. In the presence of base and copper(II) salts, it undergoes oxidative coupling to give diphenylbutadiyne.[6] In the presence of metal catalysts, it undergoes oligomerization, trimerization, and even polymerization.[7][8]
In the presence of gold or mercury reagents, phenylacetylene hydrates to give acetophenone:
- PhC2H + H2O → PhC(O)CH3
See also
References
- ^ Bordwell, F.G. (1988). "Equilibrium acidities in dimethyl sulfoxide solution". Acc. Chem. Res. 21: 456–463. doi:10.1021/ar00156a004.
- ^ Streitwieser, A. Jr.; Ruben, D.M.E (1971). "Acidity of hydrocarbons. XXXV. Equilibrium acidities of phenylacetylene and tert-butylacetylene in cyclohexylamine". J. Am. Chem. Soc. 93: 1794–1795. doi:10.1021/ja00736a045.
- ^ Kenneth N. Campbell, Barbara K. Campbell (1950). "Phenylacetylene". Organic Syntheses. 30: 72. doi:10.15227/orgsyn.030.0072.
- ^ John C. Hessler (1922). "Phenylacetylene". Organic Syntheses. 2: 67. doi:10.15227/orgsyn.002.0067.
- ^ Nwokogu, Godson C.; Zemolka, Saskia; Dehme, Florian (2007). "Trimethylsilylacetylene". EROS. doi:10.1002/047084289X.rt288.pub2. ISBN 978-0471936237.
- ^ Campbell, I. D.; Eglinton, G. (1965). "Diphenyldiacetylene". Organic Syntheses. 45: 39. doi:10.15227/orgsyn.045.0039.
- ^ Gerhard Hilt; Thomas Vogler; Wilfried Hess; Fabrizio Galbiati (2005). "A simple cobalt catalyst system for the efficient and regioselective cyclotrimerisation of alkynes". Chemical Communications. 2005 (11): 1474–1475. doi:10.1039/b417832g. PMID 15756340.
- ^ Ardizzoia, G. A.; Brenna, S.; Cenini, S.; LaMonica, G.; Masciocchi, N.; Maspero, A. (2003). "Oligomerization and Polymerization of Alkynes Catalyzed by Rhodium(I) Pyrazolate Complexes". Journal of Molecular Catalysis A: Chemical. 204–205: 333–340. doi:10.1016/S1381-1169(03)00315-7.
- v
- t
- e
aliphatic
hydrocarbons
| Alkanes CnH2n + 2 |
| ||||
|---|---|---|---|---|---|
| Cycloalkanes | |||||
| Alkylcycloalkanes |
| ||||
| Bicycloalkanes |
| ||||
| Polycycloalkanes |
| ||||
| Other |
|
aliphatic
hydrocarbons
| Alkenes CnH2n |
| ||||
|---|---|---|---|---|---|
| Alkynes CnH2n − 2 |
| ||||
| Cycloalkenes | |||||
| Alkylcycloalkenes |
| ||||
| Bicycloalkenes | |||||
| Cycloalkynes |
| ||||
| Dienes |
| ||||
| Other |
|
hydrocarbons
| PAHs |
| ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alkylbenzenes |
| ||||||||||||||||||||||
| Vinylbenzenes | |||||||||||||||||||||||
| Other |
|